The PDP model has shown its efficacy in the global response to COVID-19, which makes use of many of the attributes, human capital, and approaches core to PDPs, including innovative partnerships that share the risks and rewards of product development. Further, PDPs themselves play a critical role in preventing and responding to urgent and emerging health threats, including COVID-19, antimicrobial resistance (AMR), and pandemic preparedness. Addressing these threats promotes global development and health security.
With sufficient funding, ample political will, and partnerships across various sectors, including industry, academia, governments, communities, and multilateral organizations, PDPs can continue to develop and drive equitable global access to health technologies with the potential to save millions of additional lives, help lift people out of poverty, and improve global health security over the coming decades.
- Drugs for Neglected Diseases initiative (DNDi)
- European Vaccine Initiative (EVI)
- Foundation for Innovative New Diagnostics (FIND)
- Innovative Vector Control Consortium (IVCC)
- IAVI
- International Partnership for Microbicides (IPM)
- International Vaccine Institute (IVI)
- Medicines Development for Global Health (MDGH)
- Medicines for Malaria Venture (MMV)
- Path
- Tuberculosis Vaccine Initiative (TBVI)
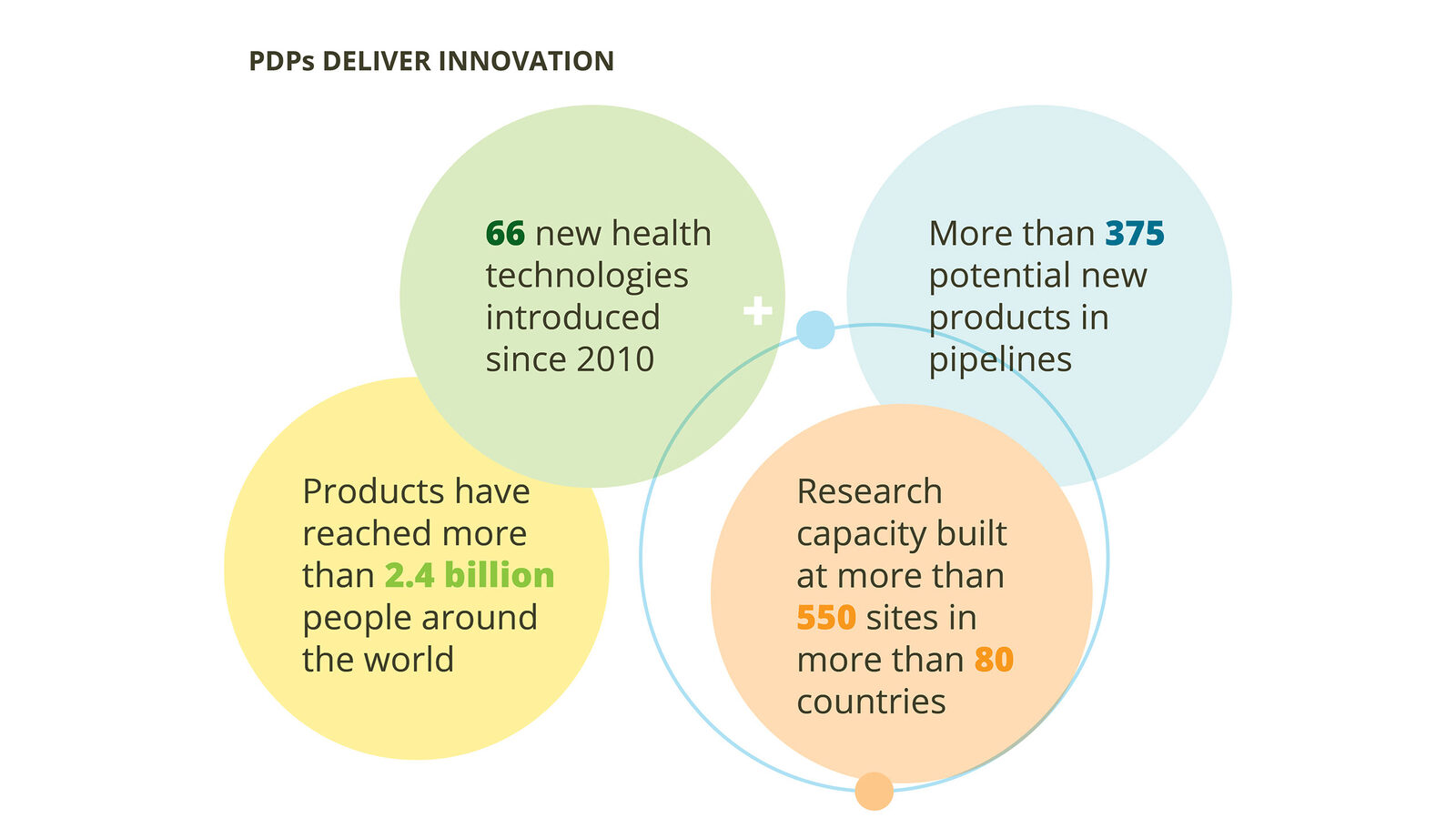
PDP products and innovation have improved health and saved lives by addressing unmet needs:
- PDPs are the global leaders in developing new health technologies where lack of traditional market incentives have stalled progress.
- PDP pipelines are robust and poised to deliver a significant number of innovative technologies in the near term.
- PDPs achieve impact by developing products appropriate for the people and contexts in which they will be used.
- PDPs save money and are a cost-effective way to save lives and grow economies.
- PDPs build local capacity to perform research and strengthen health systems.
- The products that PDPs develop are essential to achieve universal health coverage and the United Nations 2030 Sustainable Development Goals.
- PDPs are equipped to help prevent and respond to urgent emerging and future health threats.
- PDPs need sustained, diverse, and flexible funding to increase their impact on global health and development.
- Regulatory harmonization is needed to accelerate the global availability of PDP-developed products.
- Increased investment and cross-sector collaboration are needed to ensure the widespread adoption, delivery, and implementation of new health technologies.
********************
IAVI: A Case Study on translating HIV vaccine development work to contribute to new health challenges

IAVI exemplifies this flexibility as it has translated work on HIV vaccine development to identifying and developing neutralizing antibodies to combat HIV, COVID-19, and other diseases, spearheading novel partnerships and business models to ensure that these can become globally accessible. Facilitated through historic investments from its donors, including the Dutch Ministry of Foreign Affairs, IAVI is using novel technologies to identify, characterize, and optimize antibodies for potency and breadth. Further, IAVI works with global partners, including the U.S. National Institutes of Health (NIH) and the Serum Institute in India, to prepare to scale up production of broadly neutralizing antibodies (bnAbs) for HIV, and applying more sustainable, state-of-the-art, and low-cost manufacturing technologies, targeting the most desirable product profile to ensure affordability and foster ease of implementation and acceptance. A first set of these bnAbs are being evaluated in clinical trials, and next-generation versions are expected to progress to clinical development in late 2021.
These advances continue to spur biomedical innovation to develop prevention tools and treatments beyond HIV/AIDS. For example, IAVI, The Liverpool School of Tropical Medicine, and other partners, with funding from the United Kingdom’s Foreign Commonwealth and Development Office (previously DFID), are applying these techniques to develop novel antibody-based snakebite therapy as part of the five-country SRPNTS consortium.
With monoclonal antibodies (mAbs) becoming rapidly one of the most powerful tools in modern medicine, yet less applied for combatting infectious diseases, IAVI partnered with Wellcome to publish in 2020 a Global Access to Antibodies report . This analysis of the gaps and opportunities for end-to-end development and equitable global access to mAbs is an urgent call to action for leaders across the globe to realize a roadmap for global access, especially for people in LMICs, to innovative antibody-based solutions for COVID-19, HIV, and other infectious and non-communicable diseases that affect people in LMIC countries disproportionally.
This report was developed by the Coalition of PDPs and features IAVI’s work.